MS 201 Homework 1 introduction Consider the BCC unit cell shown below And then for triangle NPQ, But = 4R, R being the atomic radius. Also, = a. Therefore, (NP) 2 = a 2 + a 2 = 2a 2 (NQ)2 = (QP)2 + (NP) 2 The APF is just the total sphere volume-unit cell volume ratio. For HCP, there are the equivalent of six spheres per unit cell, and thus
Solved Question 14 The accompanying figure shows three | Chegg.com
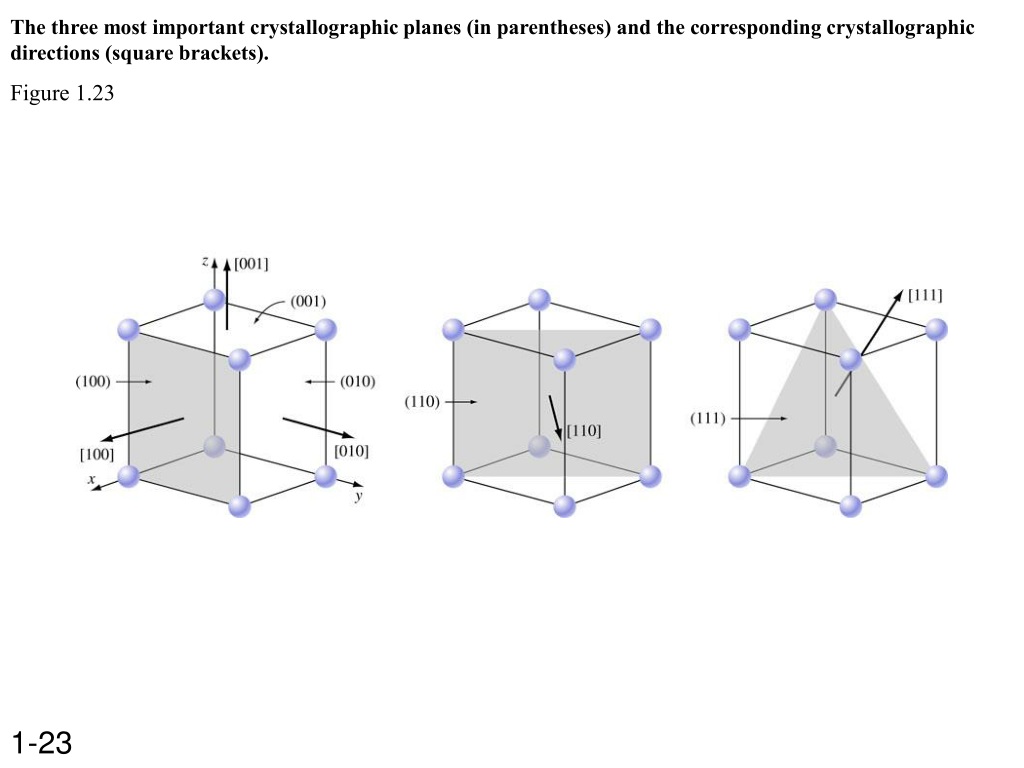
Examples Sketch the following directions : [110], [1 2 1], [ 1 0 2] uvtw ] v ‘ ) u ‘ ) Crystallographic planes Orientation representation (hkl)–Miller indices Parallel planes have same miller indices Determine (hkl) A plane can not pass the chosen origin A plane must intersect or parallel any axis

Source Image: skillshare.com
Download Image
Mar 25, 2023The accompanying figure shows three different crystallographic planes for a unit cell of some hypothetical metal. The circles represent atoms: (a) To what cr

Source Image: researchgate.net
Download Image
Hermann–Mauguin notation – Wikipedia Oct 10, 20231. First, we need to identify the three different crystallographic planes shown in the figure. These planes are defined by the arrangement of atoms in the unit cell. Step 2/4 2. Next, we can analyze the atomic packing in each plane. This can be done by looking at the arrangement of atoms and the distances between them.
Source Image: quora.com
Download Image
The Accompanying Figure Shows Three Different Crystallographic Planes
Oct 10, 20231. First, we need to identify the three different crystallographic planes shown in the figure. These planes are defined by the arrangement of atoms in the unit cell. Step 2/4 2. Next, we can analyze the atomic packing in each plane. This can be done by looking at the arrangement of atoms and the distances between them. The accompanying figure shows three different crystallographic planes for a unit cell of some hypothetical metal. The circles represent atoms. (a) To what crystal system does the unit cell belong? (b) What would this crystal structure be called? (c) If the density of this metal is 18.91 g/cm3 18.91 g / c m 3, determine its atomic weight.
Can collection techniques be used to observe and analyze crystallographic planes? – Quora
The accompanying figure shows three different crystallographic planes for a unit cell of some hypothetical metal. The circles represent atoms: 0.30 nm- 0.25 nm 0.20 nm (110) (101) (011) (a) To what crystal system does the unit cell belong? (b) What would this crystal structure be called? (c) Ifthe density of this metal is 18.91 g/cm, determine ⏩SOLVED:The accompanying figure shows three different… | Numerade

Source Image: numerade.com
Download Image
⏩SOLVED:The accompanying figure shows three different… | Numerade The accompanying figure shows three different crystallographic planes for a unit cell of some hypothetical metal. The circles represent atoms: 0.30 nm- 0.25 nm 0.20 nm (110) (101) (011) (a) To what crystal system does the unit cell belong? (b) What would this crystal structure be called? (c) Ifthe density of this metal is 18.91 g/cm, determine

Source Image: numerade.com
Download Image
Solved Question 14 The accompanying figure shows three | Chegg.com MS 201 Homework 1 introduction Consider the BCC unit cell shown below And then for triangle NPQ, But = 4R, R being the atomic radius. Also, = a. Therefore, (NP) 2 = a 2 + a 2 = 2a 2 (NQ)2 = (QP)2 + (NP) 2 The APF is just the total sphere volume-unit cell volume ratio. For HCP, there are the equivalent of six spheres per unit cell, and thus
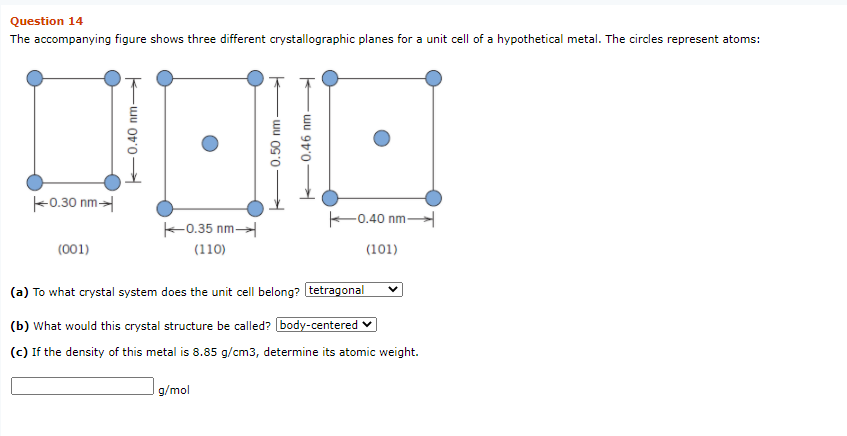
Source Image: chegg.com
Download Image
Hermann–Mauguin notation – Wikipedia Mar 25, 2023The accompanying figure shows three different crystallographic planes for a unit cell of some hypothetical metal. The circles represent atoms: (a) To what cr

Source Image: en.wikipedia.org
Download Image
⏩SOLVED:The accompanying figure shows three different… | Numerade VIDEO ANSWER: So, given in the question, the three crystallographic planes for a unit cell of a hypothetical metal are shown as follows. So, one plane is drawn like this, the other one slightly bigger than this, th … The accompanying figure shows three different crystallographic planes for a unit cell of a hypothetical metal. The circles
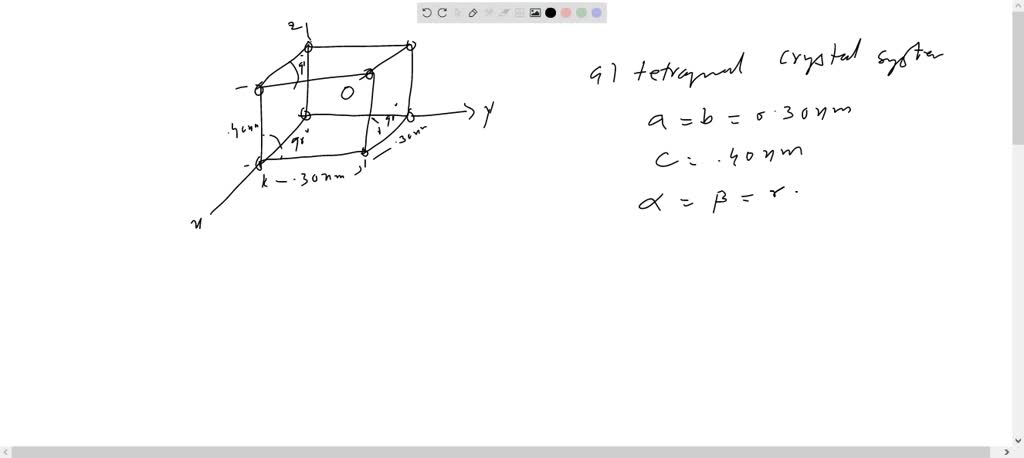
Source Image: numerade.com
Download Image
PPT – 1-21 PowerPoint Presentation, free download – ID:9729768 Oct 10, 20231. First, we need to identify the three different crystallographic planes shown in the figure. These planes are defined by the arrangement of atoms in the unit cell. Step 2/4 2. Next, we can analyze the atomic packing in each plane. This can be done by looking at the arrangement of atoms and the distances between them.
Source Image: slideserve.com
Download Image
Victor Vasarely Stri-Oet – Art Blart _ art and cultural memory archive The accompanying figure shows three different crystallographic planes for a unit cell of some hypothetical metal. The circles represent atoms. (a) To what crystal system does the unit cell belong? (b) What would this crystal structure be called? (c) If the density of this metal is 18.91 g/cm3 18.91 g / c m 3, determine its atomic weight.

Source Image: artblart.com
Download Image
⏩SOLVED:The accompanying figure shows three different… | Numerade
Victor Vasarely Stri-Oet – Art Blart _ art and cultural memory archive Examples Sketch the following directions : [110], [1 2 1], [ 1 0 2] uvtw ] v ‘ ) u ‘ ) Crystallographic planes Orientation representation (hkl)–Miller indices Parallel planes have same miller indices Determine (hkl) A plane can not pass the chosen origin A plane must intersect or parallel any axis
Hermann–Mauguin notation – Wikipedia PPT – 1-21 PowerPoint Presentation, free download – ID:9729768 VIDEO ANSWER: So, given in the question, the three crystallographic planes for a unit cell of a hypothetical metal are shown as follows. So, one plane is drawn like this, the other one slightly bigger than this, th … The accompanying figure shows three different crystallographic planes for a unit cell of a hypothetical metal. The circles